Population Pharmacokinetics of Tamibarotene in Pediatric and Young Adult Patients with Recurrent or Refractory Solid Tumors
- PMID: 39590158
- PMCID: PMC11592880
- DOI: 10.3390/curroncol31110527
Population Pharmacokinetics of Tamibarotene in Pediatric and Young Adult Patients with Recurrent or Refractory Solid Tumors
Abstract
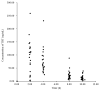
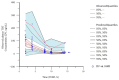
Tamibarotene is a synthetic retinoid that inhibits tumor cell proliferation and promotes differentiation. We previously reported on the safety and tolerability of tamibarotene in patients with recurrent or refractory solid tumors. Therefore, in this study, we aimed to evaluate the pharmacokinetic properties of tamibarotene and construct a precise pharmacokinetic model. We also conducted a non-compartmental analysis and population pharmacokinetic (popPK) analysis based on the results of a phase I study. _targeted pediatric and young adult patients with recurrent or refractory solid tumors were administered tamibarotene at doses of 4, 6, 8, 10, and 12 g/m2/day. Serum tamibarotene concentrations were evaluated after administration, and a popPK model was constructed for tamibarotene using Phoenix NLME. During model construction, we considered the influence of various parameters (weight, height, body surface area, and age) as covariates. Notably, 22 participants were included in this study, and 109 samples were analyzed. A two-compartment model incorporating lag time was selected as the base model. In the final model, the body surface area was included as a covariate for apparent total body clearance, the central compartment volume of distribution, and the peripheral compartment volume of distribution. Visual prediction checks and bootstrap analysis confirmed the validity and predictive accuracy of the final model as satisfactory.
Keywords: pediatric; population pharmacokinetics; tamibarotene; young adult.
Conflict of interest statement
TU received a research grant from OHARA Pharmaceutical Co., Ltd. All other authors report no potential conflicts.
Figures

Similar articles
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Comparing Three Ways to Help Cancer Survivors Plan for Follow-Up Care [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2022 May. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2022 May. PMID: 39666842 Free Books & Documents. Review.
-
Tamoxifen for adults with hepatocellular carcinoma.Cochrane Database Syst Rev. 2024 Aug 12;8(8):CD014869. doi: 10.1002/14651858.CD014869.pub2. Cochrane Database Syst Rev. 2024. PMID: 39132750 Review.
References
-
- Nakata K., Matsuda T., Hori M., Sugiyama H., Tabuchi K., Miyashiro I., Matsumoto K., Yoneda A., Takita J., Shimizu C., et al. Cancer incidence and type of treatment hospital among children, adolescents, and young adults in Japan, 2016–2018. Cancer Sci. 2023;114:3770–3782. doi: 10.1111/cas.15892. - DOI - PMC - PubMed
-
- London W.B., Castel V., Monclair T., Ambros P.F., Pearson A.D.J., Cohn S.L., Berthold F., Nakagawara A., Ladenstein R.L., Iehara T., et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J. Clin. Oncol. 2011;29:3286–3292. doi: 10.1200/JCO.2010.34.3392. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

